https://www.youtube.com/watch?v=Ev2dEqrN4i0
p.s. Antiwar continues........this may be an answer......then again, the inferno may affect the Depleted Uranium and radiation in the atmosphere also........affect the whales, sea creatures....
How's about a scientific view:
This site requires a lot of work. We hope you find our efforts valuable and rewarding. Please consider offering your support. There is no minimum amount. Feel free to donate as you see fit, without restriction. Thank you...
Nuclear Waste: Is it?
What is nuclear waste? Most people have no idea.
When asked "What is nuclear waste?", most people might answer "garbage from nuclear plants" or perhaps "the stuff that comes from reactors". As it turns out, both answers are superficially correct, but entirely miss the point. All human endeavors produce garbage. We know what we have in our household garbage, but precious few people know what constitutes nuclear waste. There are actually two classifications of the stuff; low-level and high-level radioactive wastes. Low-levelnuclear waste is essentially the trash produced from cleaning materials and plant maintenance, similar to most industrial garbage with one difference; it's detectably radioactive. Low-level waste from nuclear plants is about equal in volume and radioactivity to the combined radioactive trash from hospitals, colleges, and research laboratories. And, it's usually less radioactive than the huge, un-monitored volumes of fly ash dumped daily from coal-burning power plants. (see reference 2, below) Regardless, low-level nuclear waste really isn't the issue. High level nuclear waste is the core of the issue; specifically the fuel cells that come from the power plant reactor after they can no longer maintain an efficient chain reaction. The nuclear waste issue boils down to this high-level stuff, and misconceptions abound.
What is it?
The so-called waste atoms in the spent, exhausted fuel cells after they come out of the reactor, are the remains of the fuel's Uranium (U-235) and Plutonium (Pu-239) nuclei after they split (fission). These pieces are known as "fission fragments" in nuclear jargon. In everyday language, they are nuclear waste atoms.
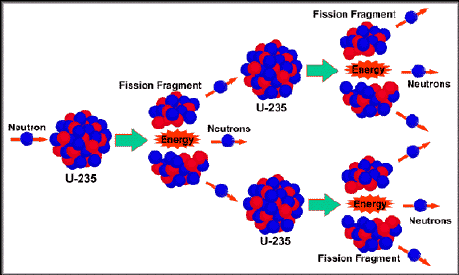
The resulting atomic fission products are newly-formed elements which are no longer U-235 or Pu-239. They have become a large number of common elements including all rare earths, a number of active metals, semi-precious metals and even a trace of Silver. Neutrons seldom spilt nuclei right down the middle. On uncommon occasions the U-235 and Pu-239 do split in half, but most of the time they don't. One thing that always happens; if you take the atomic number of one of the new atoms, let's say Cesium (atomic no. 55), and subtract it from Uranium's atomic number (92), we get the atomic number of the other new atom, which is 37. This turns out to be Rubidium. Quite simply, the atomic numbers of the two new atoms, freshly made by splitting Uranium, always equal 92. When Pu-239 fissions, the atomic numbers of the two new atoms add up to Plutonium's atomic number of 94.
As it turns out, The Cesium-Rubidium pair is the most probable result of U-235 fission, at a little less than 10% of all possible pairings. Other pairs in the 2% to 9% range include Barium-Krypton, Strontium-Xenon, and Yttrium-Iodine. The vast majority of pairings (always adding up to atomic number 92 or 94) are progressively less probable, all the way down to a tiny probability with the Iron-Dysprosium pair, at much less than .001 %. In total, a spectrum of 42 possible elements from U-235 fissions. With Plutonium, the pairings are slightly different because Pu-239 has a higher atomic number, which is two more than U-235. This adds four more possible elements, including Manganese, Chromium, Holmium and Erbium, all of which will have a fuel cell abundance of less than .01 %. In all cases, these freshly made elements are all immediately radioactive.
The most intensely radioactive waste elements are those with the shortest half-lives. The least radioactive waste elements are those with the long half lives. The ones we really need to concern ourselves with are those with half-lives between one day and five billion years. All radioactive materials will lose their easily detectable radioactivity after about nine half-lives. To be conservative, let's say the radioactive lifetime of a radioactive element is ten half-lives. Any isotope with a half life less than a day is essentially non-radioactive after 10 days, and is of no long-term consequence. It takes longer than 10 days after reactor shutdown to begin moving the used fuel out of the reactor for long-term storage, so the very short half-life isotopes are not an issue. At the converse extreme, all isotopes with a half life greater than 5 billion years are so low in actual radioactivity that it becomes difficult to distinguish them from totally stable, non-radioactive elements. Because of their minuscule radioactivity, they are also of no long-term consequence. Interestingly, some naturally-occurring elements are so subtly radioactive that their unstable nature was not understood until about 35 years after Hiroshima, when detectors of ultra-high sensitivity first became available. These subtly radioactive natural elements include (but are not limited to) Indium-115, Tellurium-130, and Lanthanum-138, all with half-lives of 10 billion years or more, and none of which are considered radioactively significant. All three are also to be found in the nuclear waste matrix.
Numerically, about 5% of the waste elements made from splitting U-235 and Pu-239 have half lives less than one day. Another 75% of the radioactive elements we find in high level nuclear waste have half lives between one day and five years. We thus find the majority of the waste elements are non-radioactive after about 50 years. This includes many valuable rare earths such as Neodymium, (welders goggles, light-spectrum calibration equipment in astronomy, and laser technology) and Ruthenium (low cost solar cells). Other modern uses of these Rare Earths include magnets in hybrid cars, wind turbines, computer hard drives and cell phones. There are also many active, semi-precious metals in the waste atom matrix such as Cadmium, which is used in making batteries and electroplating. Recycling exhausted power plant reactor fuel after 50 years of closely-monitored storage would make these resources available to the world. Burying un-recycled fuel cells would be throwing these valuable materials away, which would be a true waste.
In fact, only 8 of the nuclear waste elements have isotopes which are of radioactive concern after 50 years. Only 3 of the 15 Rare Earths in nuclear waste are detectably radioactive after 50 years (Promethium, Gadolinium, and Terbium). Recycling could remove these long-lived radioactive elements from the non-radioactive matrix and placed back into storage. But, should we not just throw these remaining radioactive residuals away? Of course not, because of the 3 valuable Rare Earths and a bit of Silver in there (which has the longest isotopic half life of about 100 years), plus four other valuable active and semi-precious metals. Eventually, these precious materials can be recovered and become a valuable resource to our future descendants. Patience is, after all, a virtue.
Although almost never mentioned, some of the radioactive elements in exhausted fuel cells with half lives a bit greater than a few weeks are very useful in medical healing practices. These include specific isotopes (atomic varieties) of Cesium, Strontium, Yttrium, Iodine, and Xenon. By recycling exhausted fuel within a few months after removal from a reactor, these valuable medical tools could be available. This does not mean exhausted reactor fuel ought to be recycled such a short time after reactor removal. However, even calling freshly made exhausted fuel a "waste" is far from correct.
In actuality, by making "nuclear waste atoms" we're literally realizing the old alchemist's dream of turning crude base metal into something precious. Split U-235 and Pu-239, recycle the spent fuel after 50 years, and we get lots of valuable stuff. As it turns out, by removing all of the so-called waste atoms from the exhausted fuel, and we do nothing other than bury the stripped fuel cells, the remaining fuel cells becomes less toxic than natural Uranium in less than 500 years! That's a long time, to be sure, but not the thousands-upon-thousands of years routinely expounded on through the news media and preached about by many governmental bodies. By not recycling the fuel, it remains more toxic than natural uranium for about 100,000 years. That's a very, very long time, but it need not be the case!
"It'll be radioactive forever!"
Another myth has to do with the radioactive toxic lifetime of high level nuclear waste. It won't be forever. The entire universe will be radioactive forever due to the perpetual production of radioactive isotopes by stars. But, not high level nuclear power plant waste. Here's the cold, technical facts.
Brand new Uranium fuel cells are made up of naturally-occurring U-238 and U-235 atoms, with 97-99% of the matrix U-238 and 1-3% U-235. Exhausted reactor fuel, after about three years in the reactor, is about 5% waste atoms, 1% Plutonium isotopes, and 94% U-238, when it is removed. The natural Uranium-238, which the waste atoms and Plutonium are encased in, has a half-life of 4.5 billion years. The "it'll be radioactive forever" notion comes from the natural U-238 making up the large majority of the exhausted fuel with an effective lifetime of 45 billion years. The reactor fuel had essentially a 45 billion year radioactive lifetime when it came from the Earth, thus it was "radioactive forever" before it was ever installed in the reactor. Un-recycled spent fuel cells will actually be less radioactive than natural Uranium after roughly 500 years because more than 99% of the "waste" atoms will have completely lost their radioactivity, and the half life of the Plutonium atoms is considerably less than U-235 so it diminishes total radioactivity much faster than naturally-occurring Uranium. Since the waste atoms and the exhausted fuel itself is not actually a "radioactive forever" issue, what's the problem?
The BIG Problem...or is it?
Plutonium is most often perceived to be the problem. For the first 5 decades of the post-Hiroshima era, Plutonium was believed to be only a man-made element with but one use…making bombs. All Plutonium was assumed to be "weapon's grade" simply because it could hypothetically be used to make bombs, thus the best thing to do would be to bury the exhausted fuel cells from power plants a mile or two deep in the Earth, in solid rock or salt deposits, and leave it there. The technical realities with power plant Plutonium are very different from these essentially fictitious beliefs.
In the late 1990's, Plutonium was discovered to exist in trace quantities of a few unusual natural Uranium deposits around the world. U-238 may not fission in a reactor environment, but it does have a very small probability of spontaneous fission. That is, on rare occasion, an atom of U-238 will split apart all by itself. Each spontaneous U-238 fission releases three neutrons into the surrounding Uranium deposit. The surrounding U-238 atoms absorb many of these fresh neutrons and become U-239. U-239 radioactively decays rather quickly. This is because one of the neutrons in it's nucleus will literally spit out an electron (Beta radiation) and become a proton, making it a new atom of Neptunium-239. Np-239 also radioactively decays in the same way U-239 does, releasing an electron out of its nucleus and it becomes Pu-239. This only seems to happen in unusually pure natural uranium deposits. The discovery of trace amounts of naturally-occurring Pu-239 in these unusual Uranium deposits made essentially no impact on the news media, public, or the scientific community at large. But, it did make an impact on atomic scientists, and served to answer a troublesome atomic question that had plagued nuclear scientists for decades.
Where did naturally-occurring U-235 come from, anyway?
It doesn't seem to be found anywhere but in a Uranium deposits here on Earth. U-238 is sometimes found in cosmic rays but no U-235, and, many non-Uranium ores (such as coal) have trace amounts of U-238 but no U-235, indicating there is something strange about U-235 being found on Earth at all. Then the (above) discovery of natural Plutonium was made. Also, another remarkable discovery occurred. During nearly a half-century of military breeder reactors producing a significant stockpile of Plutonium, some of the stored Plutonium literally sat around for decades awaiting its use in weapons. During that time, something weird happened. The expected concentrations of Pu-239 and U-235 in the "old" stockpiles were off a tiny bit. There was a bit too much U-235, and a bit too little Pu-239. As it turns out, Pu-239 radioactively decays by releasing the nucleus of a helium atom (Alpha radiation) out of its nucleus, and becomes U-235! These two discoveries gave atomic scientists a possible reason for the existence of U-235 in nature.
When Earth formed, ~4.5 billion years ago, there must have been a primordial matrix of about 50% U-238 and 50% Pu-239 occurring, all uniformly mixed together. Pu-239 has a half-life of ~24,000 years. After about a quarter of a million years, all the Pu-239 had become U-235. U-235 has a half-life of ~700 million years. In the 4.5 billion years since the Earth was formed, the U-235 has undergone about 6.5 half-lives, resulting in the 0.7% abundance we find today. Thus, when we realized the existence of trace amounts of Pu-239 found in a few natural Uranium deposits, and the existence of Plutonium's daughter element U-235 in Plutonium stockpiles, we found that Plutonium is, was, and always will be a naturally-occurring element.
The "weapon's grade" myth
Yet, there is the stigma of the term "weapon's grade" that is routinely attached to power plant reactor-made Plutonium, which must be addressed. Let's ask a strange-sounding question. Is it really weapon's grade? Can power plant Plutonium actually be used to make a nuclear weapon?
When a new fuel cell goes into a large, relatively modern reactor (those built between 1975 and 1987), it is about 1% U-235. When a fuel cell leaves the reactor after it's 3-year "lifetime", there are almost no U-235 atoms left in it, but there is the 5% "waste" atoms and about 1% Plutonium found in its place. The 5% "waste" atoms tell an interesting story. Only about 20% of them could have possibly come from U-235 fissioning. 1% Uranium cannot make 5% waste atoms. The numbers just don't add up. Where did the other 80% of the waste atoms come from? Plutonium fissioning! While the initial loading of U-235 fissions, the U-238 is making Plutonium about as fast as the U-235 is being split. As it turns out, some 80% of the energy each fuel cell makes during it's lifetime comes from the splitting of Plutonium. Further, the 1% Plutonium in the spent fuel coming out of power plant reactors is no more "weapon's grade" than the 1% U-235 encased in fuel that originally went into the reactor. Spent fuel containing Plutonium is no more "weapon's grade" than a brand new fuel cell before it goes into a reactor.
But, there's yet another problem with calling power plant Plutonium "weapon's grade". About a third of the Plutonium made in power plant reactors is not the Pu-239 used to make bombs! A third of the Plutonium won't work in bombs.
Two isotopes of Plutonium are being formed in power plant reactors, Pu-239 and Pu-240. Pu-240 atoms naturally experience two additional neutron absorbtions and become Pu-242, which radioactively decays by Alpha emission and becomes U-238. Back to square one, if you will. The production of Pu-240 eventually plateaus at 0.32% abundance, and the Pu-239 at 0.68% abundance in the exhausted fuel cell. It's all good for reactor fuel. But, it's junk for bombs. A mixture of a 100% pure matrix of power plant Plutonium, at 68% Pu-239 and 32% Pu-240, can never explode because Pu-240 doesn't fission. Further, Pu-240 absorbs neutrons better than Pu-239. As a result, Pu-240 is what might be correctly termed a "bomb poison", because it absorbs neutrons sufficiently to keep the chain reaction from becoming extreme enough for an explosion. All by themselves, these two reasons are why power plant reactor Plutonium can not be correctly termed "weapon's grade". Together, they make the term "weapon's grade" grossly inappropriate for power plant Plutonium.
Thousands of years, and no carbon footprint
Uranium is mostly useless other than in reactors, armor for mechanized military vehicles, and bombs. Plutonium is only useful for reactor fuel and bombs. Every nucleus of U-235 and Pu-239 split in a reactor is one less atom potentially used in a bomb. Let's get rid of these bomb-possible isotopes and turn them into valuable resources (the "waste" atoms) that cannot be made into bombs. Let's utilize a paradigm of appropriate environmentalism and recycle (reprocess) exhausted power plant fuel cells. 94% of each exhausted fuel cell from a power plant reactor is still good fuel. By doing the environmentally appropriate thing, and recycling (reprocessing) the spent fuel from power plant reactors (after ~50 years?), we not only reclaim the valuable non-radioactive resources from the waste atom matrix, but we have a useful Uranium-Plutonium matrix for the making of new reactor fuel cells. Without recycling spent fuel, and only using each fuel cell once before discarding it forever as trash, we might have ~150 years of U-235 before we run out. Remember, U-235 is a trace isotope found in nature. However, by recycling the exhausted fuel, that useful lifetime stretches out to thousands of years…with no carbon footprint from the making of our electricity!
Think about it…
Summation:
1. The "waste atoms" in the fuel are approximately 46 newly-formed elements.
2. Most of the "waste atoms" can be realized as valuable resources through recycling.
3. Recycled reactor fuel can extend the availability of Uranium as a fuel by thousands of years.
4. Power plant Plutonium cannot be used to make a nuclear weapon.

References :
- Nuclear; Homeland Security News : National Terror Alert;
http://www.nationalterroralert.com/nuclear/; 2009 - Hvistendahl, Mara; Coal Ash is More Radioactive than Nuclear Waste; Scientific American; Dec. 13, 2007;
http://www.scientificamerican.com/article.cfm?id=coal-ash-is-more-radioactive-than-nuclear-waste - Gabbard, Alex; Coal Combustion : Nuclear Rescource or Danger?: Oak Ridge National Laboratory; Communications and External Relations Group; http://www.ornl.gov/info/ornlreview/rev26-34/text/colmain.html
- Backgrounder on Radioactive Waste; United States Nuclear Regulatory Commissionhttp://www.nrc.gov/reading-rm/doc-collections/fact-sheets/radwaste.html; August 18, 2009
- Whitlock, Dr. Jeremy; Waste Management; Canadian Nuclear FAQ;
http://www.nuclearfaq.ca/cnf_sectionE.htm; 2009 - Joint Convention on the Safety of Spent Fuel Management and on the Safety of Radioactive Waste Management; International Atomic Energy Agency; Vienna, Austria;
http://www-ns.iaea.org/conventions/waste-jointconvention.htm; 2003-2010 - Table of Nuclides: Korea Atomic Research Institute;
http://atom.kaeri.re.kr/; 2000 - Periodic Table of the Elements; Los Alamos National Laboratory's Chemistry Division;
http://periodic.lanl.gov/default.htm; updated,12/11/2003 - Chart of Radionuclides; Columbia University Department of earth and Environmental Sciences;
http://eesc.columbia.edu/courses/ees/lithosphere/labs/lab12/radionuclides/index.html - Interactive Chart of the Nuclides; Brookhaven National Laboratory; http://www.nndc.bnl.gov/chart/reCenter.jsp?z=6&n=8
- Oklo : Natural Nuclear Reactors; Office of Civilian Radioactive Waste Management; U.S. Department of Energy;
http://www.ocrwm.doe.gov/factsheets/doeymp0010.shtml; November, 2004 - Montgomery, Jerry, Phd and Rondo, Jeffery, PhD; Asymmetrical Fission Products; Unclear 2 Nuclear;
http://www.unclear2nuclear.com/asymFission.php; 2008 - Fission Fragments; Georgia State University Department of Physics;
http://hyperphysics.phy-astr.gsu.edu/Hbase/nucene/fisfrag.html; 2006 - Boyd, Rex; Radioisotopes in Medicine; World Nuclear Association;
http://www.world-nuclear.org/info/inf55.html; updated, February, 2010 - Winter, Mark; Plutonium; Web Elements, Ltd.; University of Sheffield, England;
http://www.webelements.com/plutonium/isotopes.html; updated, 2009 - Plutonium; Department of Radiation Protection; U.S. Environmental Protection Agency;
http://www.epa.gov/radiation/radionuclides/plutonium.html; February, 2009 - Cohen, Bernard, L.; Plutonium and Bombs: Chapter 13 of The Nuclear Energy Option; Plemum Press Inc.; 1990;
http://www.phyast.pitt.edu/~blc/book/chapter13.html
http://www.hiroshimasyndrome.com/nuclear-waste-is-it.html
or shall we surf in the
Great Pacific Garbage Patch
Island of trash twice the size of Texas floats in the Pacific
Category Watery Wonders, Disaster Areas
The Great Pacific Garbage Patch is the largest landfill in the world, though "landfill" isn't exactly the most fitting word for the thing that has formed in the middle of the Pacific ocean. The garbage patch, also known as the Pacific Trash Vortex or Eastern Garbage Patch, consists of 3.5 million tons of trash -- 90% of which is plastic debris -- that is swirling around between Hawaii and California.
The garbage vortex, which consists of waste such as toilet seats, camera cases and oil drums, accumulates from all over the world. As it moves further into the ocean, it becomes trapped by the strong flow of the North Pacific Gyre, the center of a large-scale system of rotating ocean currents.
The Great Pacific Garbage Patch is twice the size of Texas, while some scientists have argued that its mass has extended to twice the size of the United States. Though large pieces of waste remain intact in the patch, a majority of it is comprised of tiny bits of plastic, barely visible to the human eye. Due to the small size of the plastic and its location beneath the ocean's surface, the garbage patch does not show up in satellite images.
Scientists had suspected the existence of the plastic patch since 1988, but it wasn't confirmed until Charles J. Moore, a California-based sea researcher, stumbled upon the area in 1997 on his way home from a sailing race. Moore, who once described the area as plastic soup, released a study in 1999 indicating that the area contained six times more plastic than plankton. Recently, some samples have shown 48 parts plastic for every 1 plankton, meaning the levels of plastic concentration could have increased fivefold in just over a decade.
More than 200 billion pounds of plastic are created every year and at least 10% of it ends up in the ocean. Land-based plastic comprises 80% of the garbage patch; this plastic litter slowly makes it way from land, floating on the ocean's surface for years before reaching its final destination. From the west coast of North America, it takes roughly five years for plastic debris to travel in the ocean before getting caught in the gyre, and about one year from the east coast of Asia.
As it travels, the non-biodegradable plastic continues to disintegrate into tinier and tinier pieces, even down to the molecular level. The smaller the plastic breaks down, the higher chance it has of being ingested by aquatic organisms. Much of the larger plastic is covered in barnacles, making the area a popular destination for scientists studying the impact of plastic on the ecosystem.
https://www.youtube.com/watch?v=v2y1pE3yn6M
What is the answer? Contamination could be had from the massive man created floating mass filled with the deceased from FUKUSHIMA which could cause disease, etc.
Maybe you may have the right answer to deal with the massive headache heading this way......
aloha.







Replies